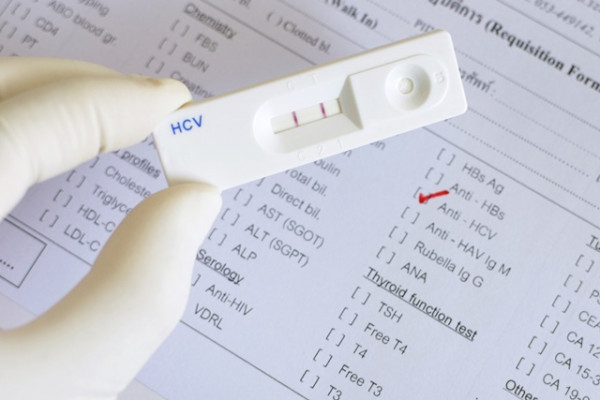
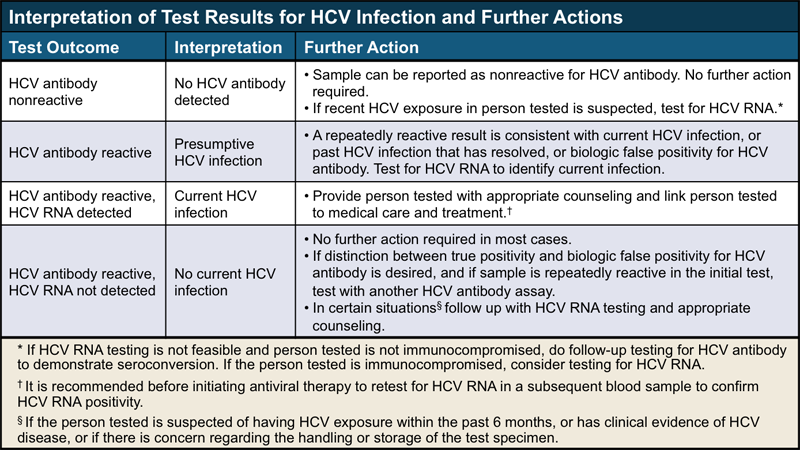
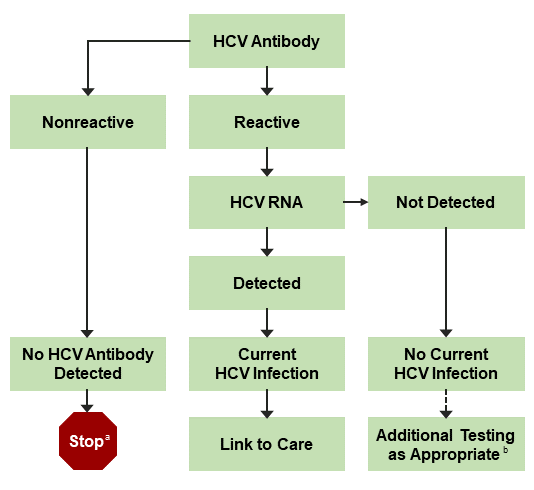
Hepatitis C Virus (HCV) Viremia in Human Immunodeficiency Virus-Seronegative and -Seropositive Patients with Indeterminate HCV R
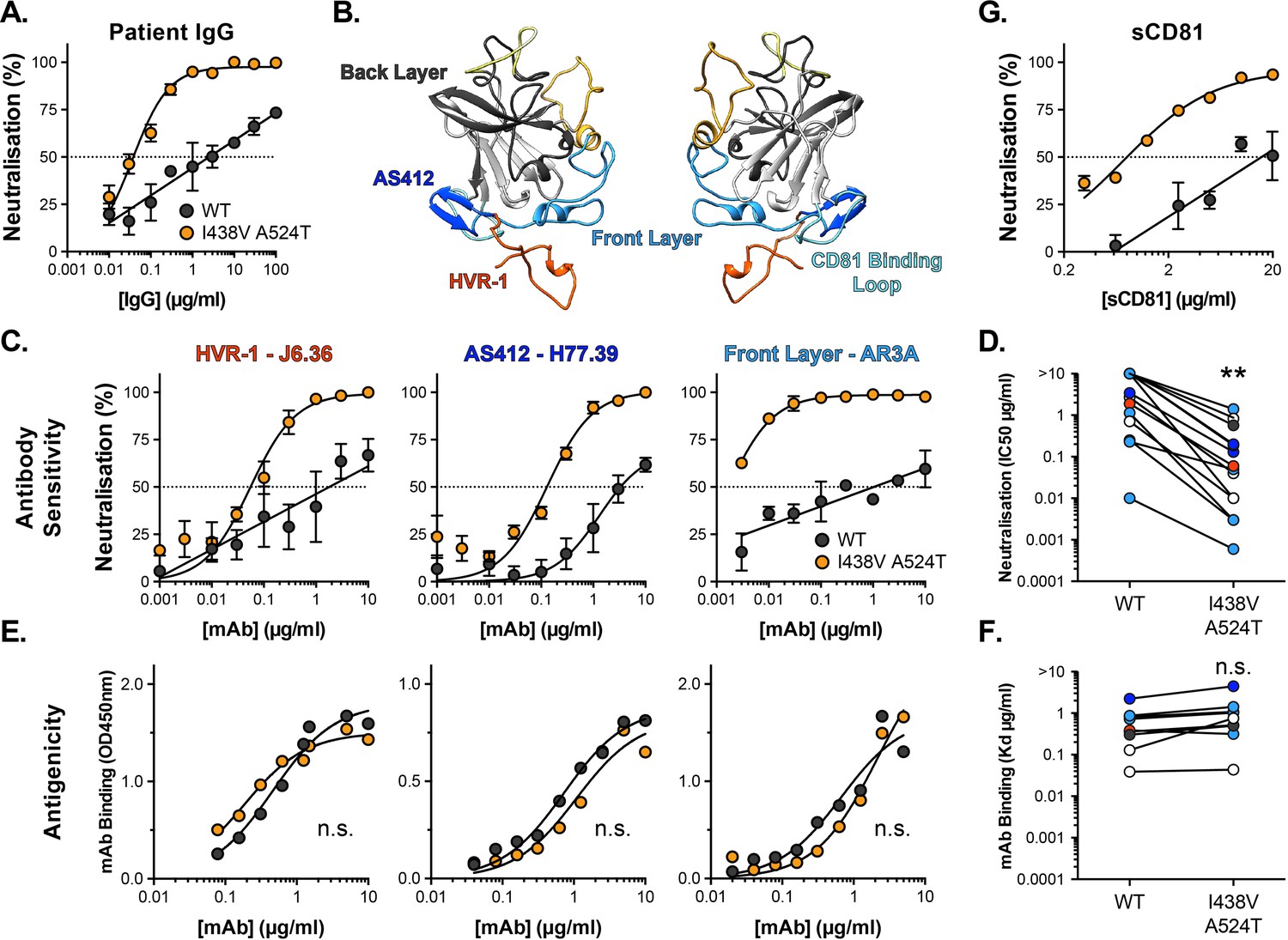
Convergent antibody responses associated with broad neutralization of hepatitis C virus and clearance of infection

PDF) Cross reactive cellular immune response to HCV genotype 1 and 4 antigens among genotype 4 exposed subjects | Sayed Abdelwahab - Academia.edu
Frequency of hepatitis C virus infection in a single institution in Mexico with a focus on birth-cohort population

Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection - Ghany - 2020 -

Hepatitis C prevalence among HIV-infected patients in Guinea-Bissau: a descriptive cross-sectional study - ScienceDirect

PDF) Hepatitis C virus RNA and liver histology in blood donors reactive to a single antigen by second-generation recombinant immunoblot assay | PAOLO BIANCHI - Academia.edu
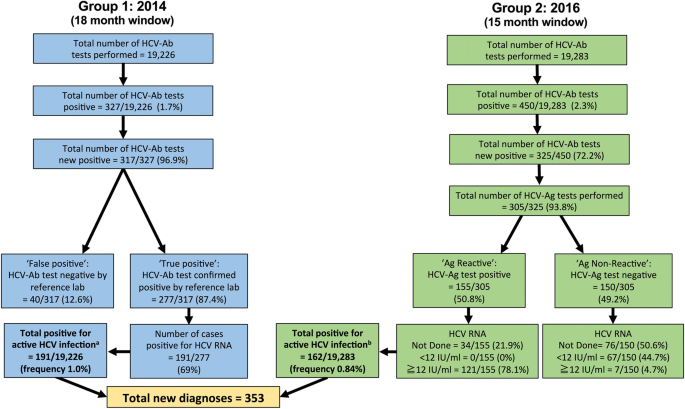
Hepatitis virus (HCV) diagnosis and access to treatment in a UK cohort | BMC Infectious Diseases | Full Text

Identification of a hepatitis C virus–reactive T cell receptor that does not require CD8 for target cell recognition - Callender - 2006 - Hepatology - Wiley Online Library
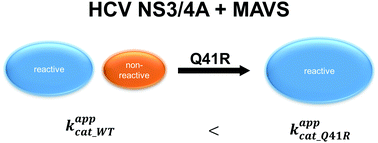
The Q41R mutation in the HCV-protease enhances the reactivity towards MAVS by suppressing non-reactive pathways - Physical Chemistry Chemical Physics (RSC Publishing)

SciELO - Brasil - Primary screening of blood donors by nat testing for HCV-RNA: development of an "in-house" method and results Primary screening of blood donors by nat testing for HCV-RNA: development
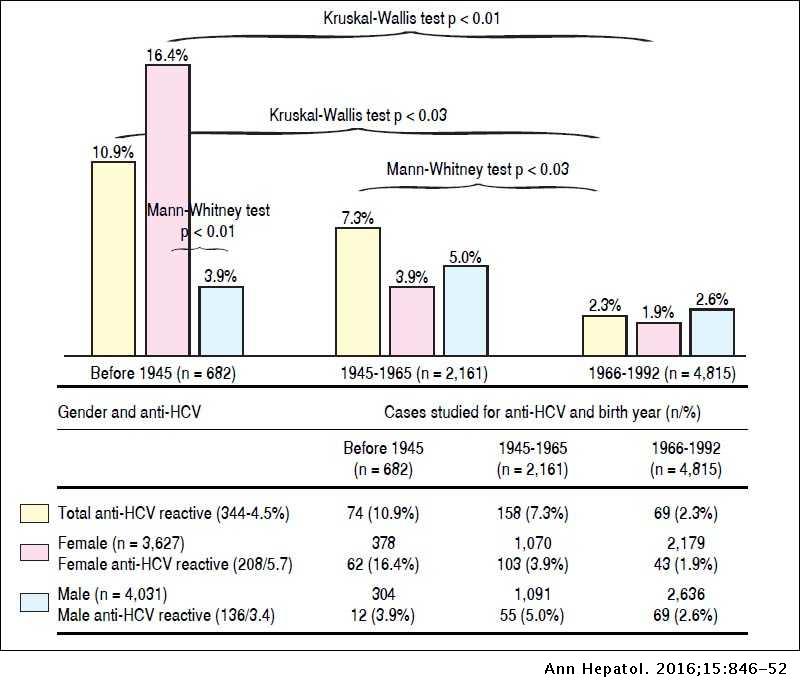
Frequency of hepatitis C virus infection in a single institution in Mexico with a focus on birth-cohort population | Annals of Hepatology

Low prevalence of hepatitis C virus RNA in blood donors with anti‐hepatitis C virus reactivity in Rwanda - Twagirumugabe - 2017 - Transfusion - Wiley Online Library